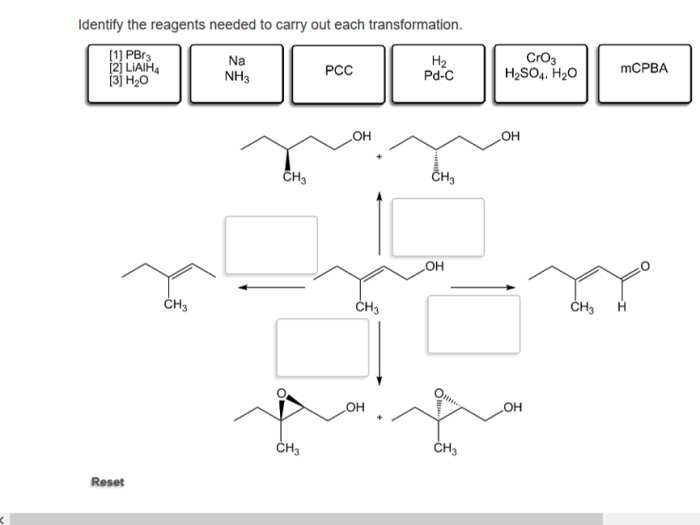
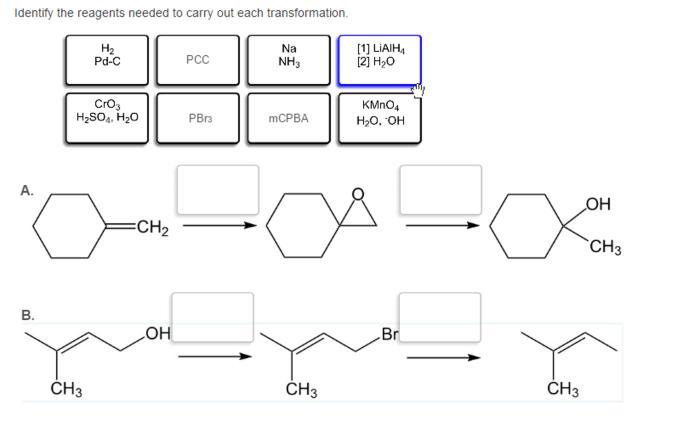
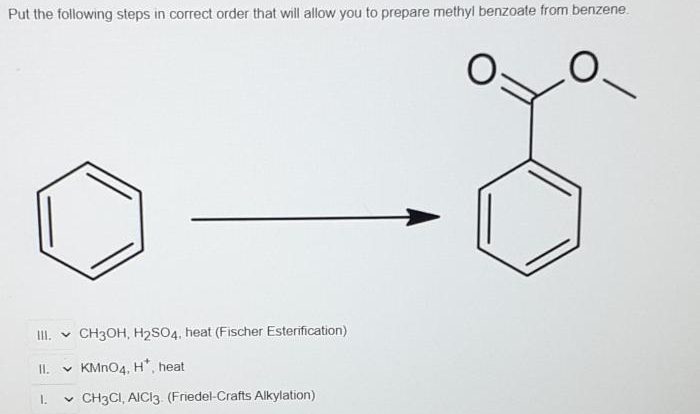
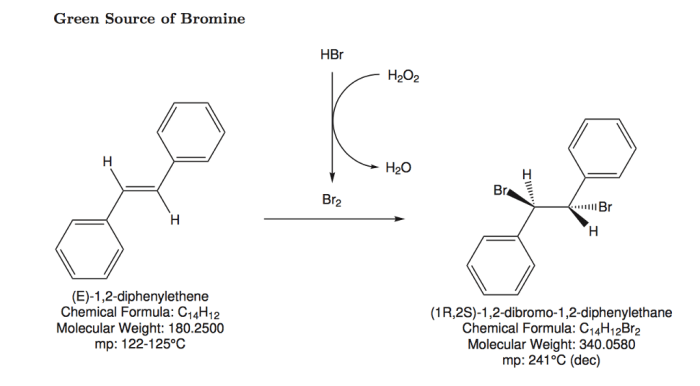
Identify the reagents needed to carry out each transformation – Identifying reagents for chemical transformations is a crucial step in organic synthesis, guiding chemists towards successful reactions and desired products. This comprehensive guide delves into the concept of reagent identification, providing a systematic approach and exploring various types of transformations and their associated reagents.
We will also delve into the significance of reagent reactivity and selectivity, practical considerations in reagent selection, and advanced techniques for identifying reagents in complex transformations.
Identifying Reagents for Organic Transformations
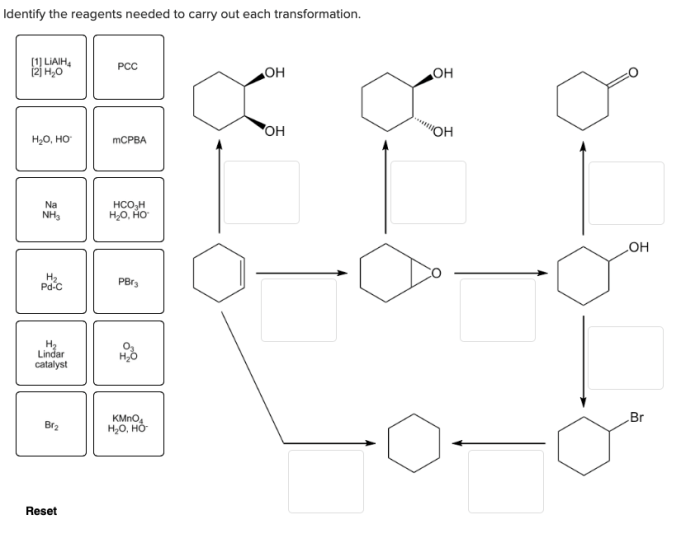
Identifying the correct reagents for a given organic transformation is crucial in organic synthesis. This process involves understanding the type of transformation, the functional groups involved, and the reactivity and selectivity of the reagents. Here’s a step-by-step guide to identifying reagents for a given transformation:
Types of Transformations and Associated Reagents
Chemical transformations can be broadly classified into various types, each with its own set of commonly used reagents. Some of the most common types of transformations include:
- Nucleophilic substitution:Reagents like alkyl halides, epoxides, and acyl chlorides are used as electrophiles, while nucleophiles like Grignard reagents, organolithium compounds, and alkoxides are commonly employed.
- Electrophilic addition:Reagents like alkenes, alkynes, and aldehydes are used as nucleophiles, while electrophiles like hydrogen halides, sulfuric acid, and epoxidizing agents are commonly used.
- Oxidation-reduction reactions:Reagents like oxidizing agents (e.g., potassium permanganate, chromic acid) and reducing agents (e.g., sodium borohydride, lithium aluminum hydride) are used to change the oxidation state of functional groups.
Reactivity and Selectivity of Reagents
The reactivity and selectivity of reagents are critical factors to consider when choosing reagents for a given transformation. Reactivity refers to the rate at which a reagent undergoes a reaction, while selectivity refers to the ability of a reagent to react with a specific functional group or regioselectively.
Choosing reagents with appropriate reactivity and selectivity is essential for achieving high yields and regioselectivity in organic synthesis. For example, in nucleophilic substitution reactions, strong nucleophiles like Grignard reagents are used for reactions that require high reactivity, while weaker nucleophiles like alkoxides are used for reactions that require regioselectivity.
Practical Considerations in Reagent Selection, Identify the reagents needed to carry out each transformation
In addition to reactivity and selectivity, practical factors like cost, availability, and safety should also be considered when selecting reagents. Cost is a major factor, especially for large-scale reactions. Availability refers to the ease with which a reagent can be obtained from commercial sources or synthesized in the laboratory.
Safety is also a paramount concern, as some reagents can be toxic, corrosive, or flammable. It is important to consult safety data sheets (SDSs) and handle reagents with appropriate precautions.
Advanced Techniques for Reagent Identification
For complex transformations, advanced techniques like retrosynthesis and computational chemistry can be used to identify reagents. Retrosynthesis involves working backward from the desired product to identify the starting materials and reagents required for the synthesis. Computational chemistry uses computer programs to predict the reactivity and selectivity of reagents, which can help in selecting the most suitable reagents for a given transformation.
User Queries: Identify The Reagents Needed To Carry Out Each Transformation
What is the significance of reagent reactivity in organic synthesis?
Reagent reactivity plays a pivotal role in determining the rate and efficiency of a chemical transformation. Highly reactive reagents lead to faster reactions, while less reactive reagents require longer reaction times or specific conditions to achieve the desired conversion.
How can I choose reagents that provide high selectivity for a specific product?
Reagent selectivity is crucial for achieving the desired product in a transformation. By understanding the mechanisms and selectivities of different reagents, chemists can choose reagents that favor the formation of the target molecule over undesired byproducts.
What are some advanced techniques used for identifying reagents in complex transformations?
Advanced techniques such as retrosynthesis and computational chemistry can assist in identifying reagents for complex transformations. Retrosynthesis involves working backward from the target molecule to identify potential synthetic pathways and reagents, while computational chemistry utilizes computer simulations to predict the reactivity and selectivity of different reagents.