Which of the following statements regarding carbon is false? Embark on an enlightening journey as we unravel the truth about this versatile element, delving into its physical and chemical properties, allotropes, biological significance, and environmental impact. Prepare to challenge your preconceptions and uncover the fascinating world of carbon.
Carbon, the backbone of life, holds a myriad of secrets waiting to be unlocked. From its ability to form diverse compounds to its role in shaping our planet’s destiny, carbon’s influence is undeniable. Join us as we explore the intricacies of this extraordinary element, separating fact from fiction and gaining a deeper understanding of its true nature.
Carbon’s Physical Properties
Carbon exhibits remarkable physical properties that contribute to its versatility and wide-ranging applications. Its physical characteristics include:
- Atomic number: 6
- Atomic mass: 12.011 amu
- Melting point: 3825 °C (graphite)
- Boiling point: 4827 °C (graphite)
- Density: 2.26 g/cm³ (graphite)
These properties play a significant role in carbon’s applications. For instance, its high melting and boiling points make it suitable for high-temperature environments, such as in furnaces and crucibles. Its low density and strong bonding structure contribute to its strength and durability, making it an ideal material for construction and engineering purposes.
Carbon’s Chemical Properties
Carbon’s chemical properties are equally remarkable, enabling it to form a vast array of compounds. Its ability to form covalent bonds with itself and other elements, known as catenation, allows for the creation of complex and diverse molecules.Carbon’s hybridization, a concept that describes the mixing of atomic orbitals to form new hybrid orbitals, plays a crucial role in determining the geometry and properties of carbon compounds.
Different hybridization states (sp, sp², sp³) lead to varying bond lengths, angles, and molecular shapes, giving rise to a wide range of carbon-containing molecules.Examples of carbon-containing molecules with varying hybridization states include methane (sp³), ethylene (sp²), and acetylene (sp). These compounds exhibit distinct chemical and physical properties due to the differences in their hybridization and molecular structure.
Carbon’s Allotropes

Carbon exists in various allotropes, which are different structural forms of the same element. These allotropes exhibit unique properties and find applications in diverse fields.The most well-known carbon allotropes are:
- Graphite: A layered structure with strong in-plane bonding and weak inter-layer bonding, resulting in high electrical and thermal conductivity and lubricity.
- Diamond: A three-dimensional network of carbon atoms with strong covalent bonds, making it the hardest known natural material.
- Graphene: A single layer of carbon atoms arranged in a hexagonal lattice, exhibiting exceptional electrical, thermal, and mechanical properties.
These allotropes find applications in various fields, including electronics, optics, materials science, and energy storage.
Carbon’s Role in Biological Systems
Carbon is the backbone of biological molecules, forming the foundation of all life on Earth. It is the central atom in carbohydrates, proteins, lipids, and nucleic acids, which are essential for cellular processes, energy metabolism, and genetic information storage.Carbohydrates provide energy to cells, proteins facilitate biological functions, lipids form cell membranes and store energy, while nucleic acids carry genetic information and control protein synthesis.
Carbon’s ability to form diverse bonds and its versatility in hybridization states enable it to fulfill these crucial roles in biological systems.
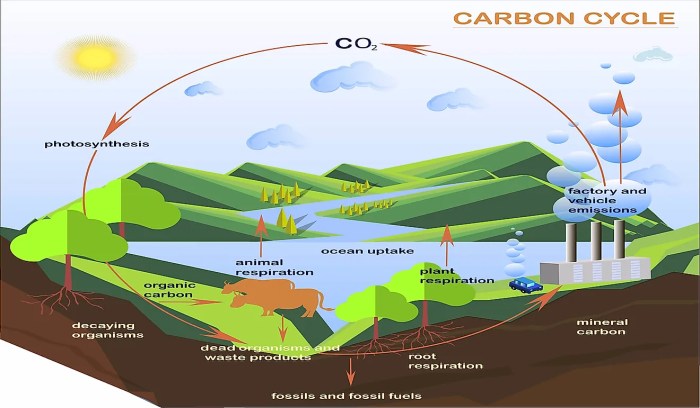
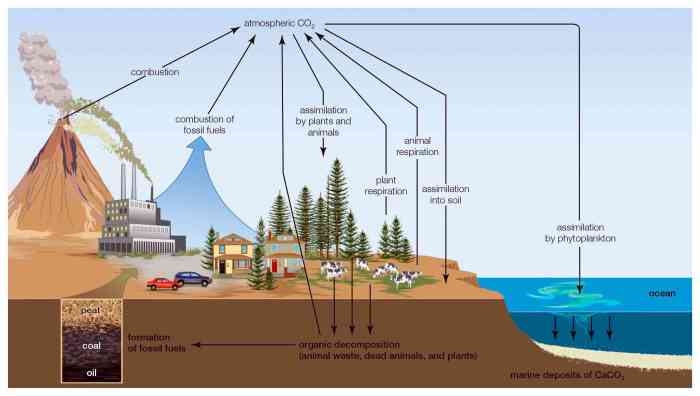
Carbon’s Environmental Impact
Carbon plays a significant role in the carbon cycle, a natural process that regulates the exchange of carbon between the atmosphere, oceans, and terrestrial ecosystems. Human activities, such as the burning of fossil fuels and deforestation, have disrupted this cycle, leading to an increase in atmospheric carbon dioxide levels.Elevated
carbon dioxide concentrations contribute to global warming and climate change, resulting in rising sea levels, changes in weather patterns, and other environmental impacts. Understanding carbon’s role in the carbon cycle is crucial for developing strategies to mitigate climate change and ensure a sustainable future.
User Queries: Which Of The Following Statements Regarding Carbon Is False
What is the significance of carbon’s hybridization?
Carbon’s hybridization plays a crucial role in determining the geometry and properties of carbon compounds. It influences the types of bonds carbon can form and the overall shape of molecules.
How do carbon allotropes differ in their properties?
Carbon allotropes exhibit distinct properties due to variations in their atomic arrangements. For instance, graphite is soft and electrically conductive, while diamond is exceptionally hard and an excellent insulator.
What is the role of carbon in cellular processes?
Carbon is the backbone of biological molecules such as carbohydrates, proteins, lipids, and nucleic acids. It plays a vital role in energy metabolism, cell division, and genetic information storage.