The mechanism for nitration of methyl benzoate unveils a captivating tale of electrophilic aromatic substitution, where the nitronium ion embarks on a thrilling journey to conquer the aromatic ring. This reaction, governed by regioselectivity and influenced by reaction conditions, finds its niche in the synthesis of a myriad of valuable products.
As we delve into the intricacies of this transformation, we shall unravel the stepwise progression of the reaction, dissect the role of the nitronium ion, and analyze the factors that dictate the preferential substitution at the ortho and para positions.
The practical applications of nitrated methyl benzoate, ranging from dyes to pharmaceuticals, will also be brought to light.
Nitration of Methyl Benzoate
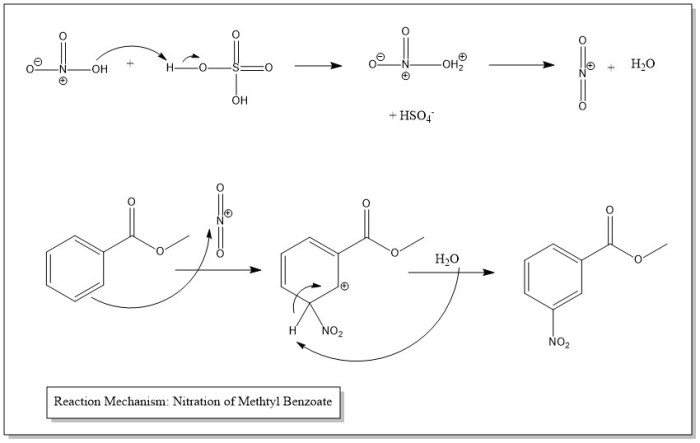
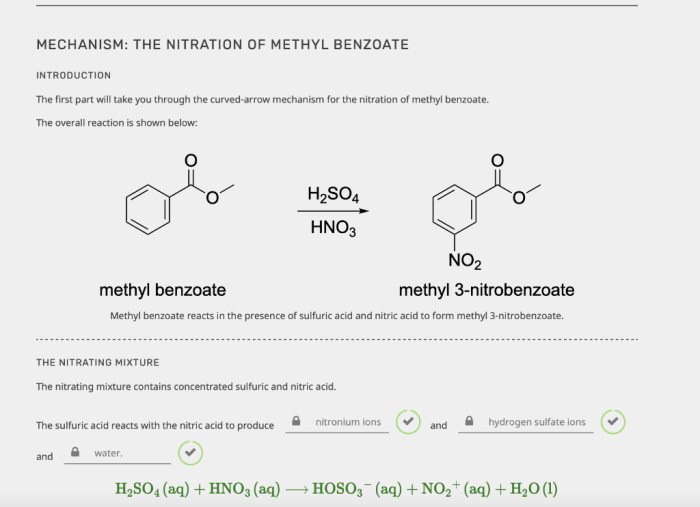
Nitration of methyl benzoate is a chemical reaction that introduces a nitro group (-NO2) into the aromatic ring of methyl benzoate. This reaction is an important step in the synthesis of a variety of products, including dyes, pharmaceuticals, and other chemicals.
Electrophilic Aromatic Substitution
The nitration of methyl benzoate is an example of electrophilic aromatic substitution. In this type of reaction, an electrophile (a species that is attracted to electrons) attacks an aromatic ring. The electrophile in the nitration reaction is the nitronium ion (NO2+).
Nitronium Ion
The nitronium ion is formed when nitric acid (HNO3) is mixed with sulfuric acid (H2SO4). The sulfuric acid protonates the nitric acid, which then loses water to form the nitronium ion.
Regioselectivity, Mechanism for nitration of methyl benzoate
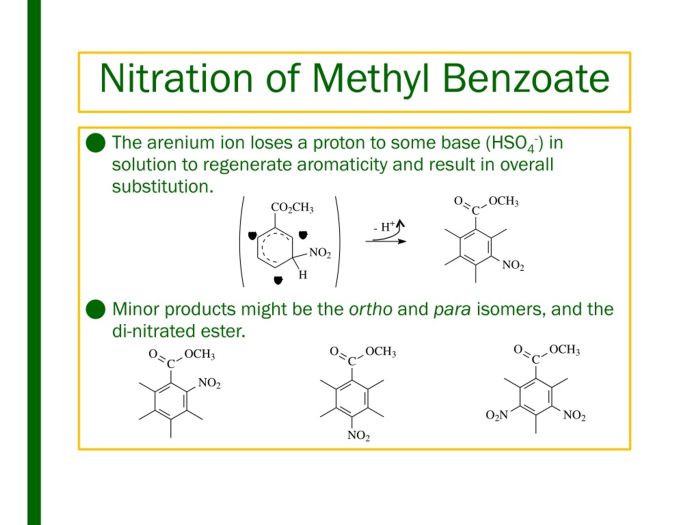
The nitration of methyl benzoate is regioselective, meaning that the nitro group preferentially substitutes at the ortho and para positions of the aromatic ring. This regioselectivity is due to the fact that the nitronium ion is a strong electrophile and is attracted to the electron-rich ortho and para positions of the aromatic ring.
Reaction Conditions
The nitration of methyl benzoate is typically carried out at room temperature in a mixture of nitric acid and sulfuric acid. The reaction can be catalyzed by a variety of Lewis acids, such as aluminum chloride (AlCl3).
Applications
Nitrated methyl benzoate is used in the synthesis of a variety of products, including:
- Dyes
- Pharmaceuticals
- Other chemicals
General Inquiries: Mechanism For Nitration Of Methyl Benzoate
What is the significance of the nitronium ion in the nitration of methyl benzoate?
The nitronium ion (NO2+) serves as the electrophile in the nitration reaction. It is generated from the reaction of nitric acid and sulfuric acid, and its electrophilic nature allows it to attack the electron-rich aromatic ring of methyl benzoate.
Why does the nitro group preferentially substitute at the ortho and para positions?
The nitro group is an electron-withdrawing group, which means it decreases the electron density of the aromatic ring. This decrease in electron density makes the ortho and para positions more susceptible to electrophilic attack, as they are less hindered and have a higher electron density compared to the meta position.
What are the applications of nitrated methyl benzoate?
Nitrated methyl benzoate is an important intermediate in the synthesis of various products, including dyes, pharmaceuticals, and explosives. It is also used as a plasticizer and a solvent.